As a new type of chromatographic stationary phase, monoliths have been subjected to intensive study in the last years. They differ from other supports mainly in their characteristic structure, which results in the improved chromatographic properties.
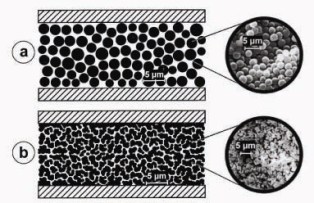
While most of the chromatographic supports are particle shaped, monoliths consist of a single piece of highly porous material. In contrast to porous particle, the pores inside the monolith are open, forming a highly interconnected network of channels. Monoliths can be prepared in various ways and can have an inorganic or an organic based skeleton [1,2,3,4,5,6].
Silica-based monoliths
The first being silica-based monolithic columns, generally prepared using sol‑gel technology. This technology can be applied to create a continuous sol‑gel network throughout the column former by gelation of a sol solution within the capillary [7,8]. Alternatively, it can be used to glue LC silica-based particles, once the capillary has been packed conventionally, producing a continuously bonded bed [9].
Organic polymer-based monoliths
The second category is rigid organic polymer-based monolithic columns, and these include acrylamide-based [10,11], methacrylate-based [12], and styrene‑based polymers [13]. The polymer network is generally formed inside the capillary by a step-wise chain polymerization reaction.
Polymerization reaction mixtures usually consist of a combination of monomers and cross-linker, initiator and a porogenic mixture of solvents. A variety of monomers can be employed to fabricate the final monolith, being both charged and hydrophilic, to generate electroosmotic flow for capillary electrochromatography, or uncharged and hydrophobic, to allow reversed-phase interactions used in HPLC. The cross-linker concentration can be adjusted to change the degree of cross-linking which influences the overall porosity. An initiator is needed to begin the step-wise chain reaction, and it is often 2,2’‑azo‑bis‑isobutyronitrile (AIBN). The polymerization can be initiated using UV light or thermal treatment.
Precipitation of the polymer occurs after it becomes insoluble in the reaction medium. Solubility is influence both the cross-linking and choice of porogen (a poor solvent for the polymer), which is commonly a mixture of alcohols.
The formation of the monolith can be achieved in-situ within either untreated or pre-treated capillaries. The pre-treatment of the capillary often involves surface preparation for the introduction of double-bond functionality, allowing covalent bonding of the monolith to the capillary wall, which is of particular importance for HPLC application where the monoliths needs to withstand high pressures.
Preparation of organic polymer monoliths
The polymerization mixture is forced into the capillary and generally initiated thermally. The reaction then continues by free radical polymerization to form a macroporous rigid monolithic polymer. The unreacted components, such as porogenic solvents, are then washed away.
References
- S. Hjerten, J.-L. Liao, R. Zhang, J. Chromatogr., 473 (1989) 273.
- T.B. Tennikova, B.G. Belenkii, F. Svec, J. Liq. Chromatogr., 13 (1990) 63.
- M. Merhar, A. Podgornik, M. Barut, M. Zigon, A. Strancar, J. Sep. Sci., 26 (2003) 322.
- H. Zou, X. Huang, M. Ye, Q. Luo, J. Chromatogr A, 954 (2002) 5.
- A.-M. Siouffi, J. Chromatogr A, 1000 (2003) 801.
- E. F. Hilder, F. Svec, J. M. J. Fréchet, J. Chromatogr A, 1044 (2004) 3.
- K. Nakanishi, N. Soga, J. Am. Ceram. Soc., 74 (1991) 2518.
- K. Nakanishi, N. Soga, J. Non-Cryst. Solids., 139 (1992) 1.
- R. Asiae, X. Huang. D. Farman, Cs. Horváth, J. Chromatogr. A., 806 (1998) 251.
- S . Hjerten, J.-L. Liao, J. Chromatogr. 457 (1988) 333.
- F. M. Plieva J. Andersson, I. Y. Galaev, B. Mattiasson, J. Sep. Sci., 27 (2004) 828.
- F. Svec, J. Sep. Sci., 27 (2004) 747.
- H . Oberacher, C.G. Huber, TrAC 21 (2002) 166.
One reply on “Monolithic stationary phases”
[…] During the time I spent in Berkeley I had the honor to work on the beginning of the project leading to the portable system for highly sensitive multi-dimensional chemical analysis. This work included hyphenation of NMR with liquid chromatography separation using organic polymer monoliths. […]