When two do the same thing, it’s not always the same. In the last issue of LCGC North America, Ronald Majors summarized his highlights of HPLC 2010. In my HPLC 2010 flashbacks I focused exclusively on oral presentations I have attended during the meeting. Ronald Majors summarized whole symposium in a detailed and comprehensive way, including the comparison of attendance this year vs. last year, percentage distribution of individual topics, as well as plenary lectures, major applications and usage of various detection techniques.
New trends in analytical chemistry
Couple of days ago I wrote about September Discussion group organized by CASSS International separation science society. As I promised I made notes and I would like to share them with you now.
Although I expected slightly different format of meeting (selection of few topics and their detailed discussion with the attendants) I was able to find some new ideas and directions in current and future analytical chemistry.
The speakers (Robert Stevenson, Tom Jupille, and David Sparkman) presented their views about new directions in analytical chemistry, liquid chromatography, and mass spectrometry.
New directions in separation science
 In May, I announced that CASSS organizes a discussion group about consulting.
In May, I announced that CASSS organizes a discussion group about consulting.
Today, I would like to mention next discussion group focused on New directions/developments in Separation Science.
The meeting is taking place on September 15th, 2010 in Woodfin Suites in Emeryville, CA at 6 pm.
Personally, I am looking forward to attending this meeting. The topic as well as a list of panelist (see below) are very interesting. It will be nice to hear opinions about new directions in separation science from people with different background and experience.
I have to admit that my knowledge, interest, and predictions focus mainly on liquid chromatography (and monoliths, of course;) so I am very curious about other topics, such as sample preparation, miniaturization and/or new materials in separations, which are possibly going to be part of the discussion too (I don’t know, just guessing;)
The information from CASSS website
Today’s sophisticated separation and analytical instruments and techniques bear scant resemblance to the “absorption analysis” technique reported by M.S. Tswett in 1903. But that same drive for innovation and improvement is alive and well in 2010.
Join us for a lively discussion on the latest trends and technical innovations presented at the Pittcon, ASMS and HPLC conferences this year. Question our expert panelists on where the trends might be taking the industry – and what to watch out for.
One thing is constant in this field … change. From mergers and acquisitions to plenty of new products – stay abreast of the trends that will affect you most.
Invited panlists
- Tom Jupille, LC Resources
- O. David Sparkman, University of the Pacific, Stockton
- Robert Stevenson, R. Stevenson Consulting
Registration starts at 5:30 p.m., dinner at 6:00 p.m., and Panel discussion at 7:00 p.m.
The prize for registration before Wednesday, September 8 is $35 for Discussion only and $49 for Discussion and Dinner. On-site registration are not eligible for dinner. More info on CASSS website.
Future post
I will try to remember (ie. make notes of;) discussed topics and possible conclusions and bring them to you here on chromatographer.com soon after the meeting. Keep in touch.
PS: if you would like to be informed about new posts, you might consider subscribtion to RSS chanel or email newsletter.
Recently, I was browsing amazon.com products related to the chromatography keyword. To my big surprise, the number ten (at least in my results) was a “16 oz. Double Wall Insulated Tumbler with chromatography column alone – Paper Insert
”.
This is what I call a present for a chromatographer!
I waited until our next order (Harry Potter Playstation 3 game for my wife) and included the coffee tumbler in the order. Partly because I didn’t (want) understand, partly because of wishful thinking but I thought that the (chromatographic) paper is inserted in between the tumbler’s walls. It wasn’t. My bad. Instead, there was inserted paper with the picture of chromatographic column (ehmm, old fashioned burette). As advertised.
Anyway, I have decided to modify the tumbler to way I see it – original tea/coffee cup with the chromatographic separation on it. My only next condition was to avoid using any laboratory equipment.
So – if you are interested – you can very easily repeat the experiment in your kitchen and prepare your own original coffee cup.
To summarize, the aim of this small experiment is to perform thin layer chromatography of black office marker on a paper as stationary phase and use this paper as an original sign of a tea/coffee tumbler. No one else will touch it and everyone will ask about the way how to do it.
Ok, that’s plan.
A little bit of theory
The black markers usually contain more then a black color with several basic colors. Therefore, the black line traced on the filtration paper immersed with one side in the mobile phase is drifted towards the other side via capillary forces. During this journey the marker’s pigments are separated into the individual colors. Pretty much as a principle of thin layer chromatography ;-) Let’s start.
Materials
For our experiment we need: black marker as a sample, filtration paper as a stationary phase and some mobile phase. Further you might need a glass, cup or pot, pencil, scotch-tape, and scissors. That’s pretty it.
Preparation of a stationary phase
As a stationary phase I have used filtration paper from our lab. This is only one violation against my no lab staff condition. The filtration paper from the coffee machine can be successfully use too. We just don’t have any. The filtration paper I have had was smaller than the paper inserted in the tumbler, thus I used two of them and cut a right size and shape according the original paper.

The reaction vessel
An empty glass. Or a cup, pot; whatever fits your paper and purpose. Better if you can have one with a lid. With the lid, vapor of your mobile phase fills vessel and speed up and improve the separation. In my case, I have used an ordinary kitchen glass (made by ikea).
Sample
As a sample I have used black Expo Vis-à-vis wet erase marker. I have tested two different markers: Expo and Sharpie. The reason why I used the Expo is that with Sharpie I didn’t get a nice separation.
Hint #1: you better try the separation before the one you want to use in your tumbler. In this case, you can select the marker you like the most.
From analytical point of view, this can be used to indentify unknown marker: just compare their traces.
Mobile phase
That’s my favorite part. As a mobile phase I have used vodka. I told you: no lab staff ;-) You can probably use any distilled brandy. I would prefer the transparent one since I am not sure about the color of (evaporated) whisky on the stationary phase paper. Since the vodka (or any kind of home made brandy) is in the range of 40 – 50% the further dilution is not necessary. In lab I would use acetonitrile : water mixture (70 : 30 or 80 : 20 ratio). The concentration composition of this mobile phase can vary depending on the desired speed and resolution of separation.
Home-made thin layer chromatography
Ok, all stuff is ready. Let’s go. First, I labeled the paper with the marker. The length of your line is up to you. You would prefer either the long line through the width of the paper or short line covering only small part of the paper. I made a 5 cm (2”) long line roughly 2.5 cm (1”) from the edge of paper. This side (under the line) is then going to be immersed in the mobile phase.
Hit #2: To help paper fits the glass I curled the paper and used hairpins to hold it. Use new ones. Otherwise you can very easily get dirty paper.
Finally, to hold the paper in a vertical position I have used a pencil. You should have avoided any touch of the paper and glass wall. The mobile phase then flows equally without any restrictions, dispersions or speed ups.
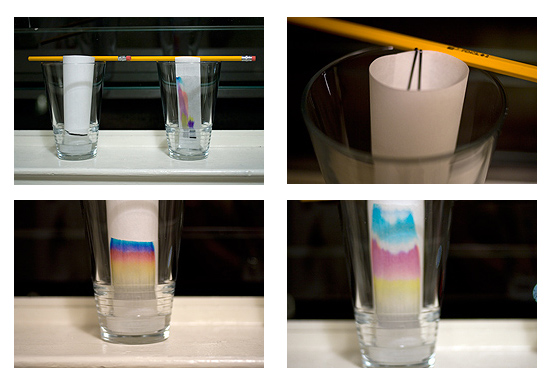
After immersing the paper in your favorite mobile phase, the liquid starts to rise via capillary forces and takes a sample with it. The low retained colors are faster than the more retained ones and “run” towards the opposite end of the paper faster. In our case you can see almost immediately after a start quick separation of four colors: black, yellow, red, and blue. As separation continues, the colors are separated more and more and later on you can notice total separation of least retained blue color from other colors. When the mobile phase reaches the other end of the paper, the separation is done. To cover only specific space of the paper, you might wish to stop the run sooner. All you need to do is then remove paper from the glass.

When the separation is finished, move the paper to other glass and let it dry. Your original sign of chromatographic society membership is ready for use.
Have fun and enjoy your coffee, tea or any kind of tasteful mobile phase.
About me

Every thing you always wanted to know about monoliths
… but were afraid to ask.
You don’t have to be afraid any more. Really, if you have any kind of question related to (mainly organic polymer based) monolithic stationary phases, please do not hesitate and send me the puzzle.
The quickest way is to use contact form. Alternativelly, you might sing up for my newsletter and you will never miss exclusive email-only information.
So, what are you waiting for? :)
More than decade with monoliths
I started to work with monolithic stationary phases in 2000 as a new member of the group of Prof. Pavel Jandera at University of Pardubice, Czech Republic. The topic of my master thesis was Preparation and characterization of capillary based monolithic columnsfor HPLC and at that time I was able to prepare and chracterize whole three columns in the time range of six month. Sweet beginnings ….
Half of Ph.D. study abroad
After my master study I continued as a Ph.D. student (again in the group of Prof. Jandera) and I was lucky enough to spend half of my Ph.D. abroud in the labs of well known and respected scientists (Prof. Mike Cooke at Royal Holloway University of London, Dr. Henk Claessens and Prof. Cor Koning at Technical University Eindhoven, Prof. Peter Schoenmakers at University of Amsterdam, and Dr. Didier Thiébaut at ESPCI ParisTech). You can find more information together with topics I have done abroad in my curriculum vitae.
The dreams came true

Since the beginning of my work with monolithic phases I found a lot of interesting and groundbreaking articles written by Frantisek Svec at University of California Berkeley. It would be nice to spend few months in his lab were my (very deep) thoughts and wishes. After few years of dreaming and few years of finishing my Ph.D. the dreams came true and from 2009 to 2011 I could spend two very nice and unforgettable years in his group in Berkeley. Never give up your dreams!
Back to beginnings
Now I am back in Czech Republic and I try to continue in what I have learned in Berkeley and whole Europe. I hope I will be able to prepare columns with completely new properties suitable for any kind of tailored separation.
Again, I hope that dreams come true. Eventually.