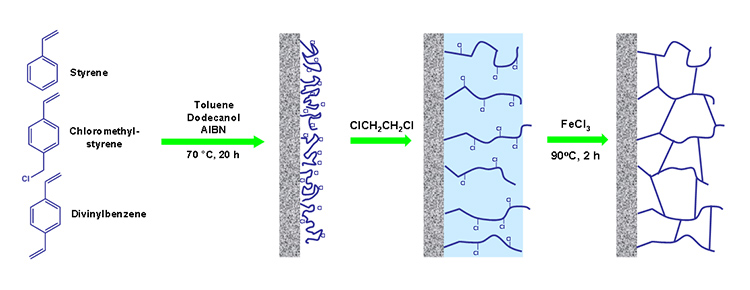
Generally, methacrylic acid is used as a charge-bearing agent for generation of electroosmotic flow in capillary electrochromatography. However, methacrylic acid has a significant effect on the morphology of the monolithic stationary phases based on styrene – divinylbenzene system as showed recently by group in Prague.
The monolithic material prepared without methacrylic acid in the polymerization mixture showed a very low surface area of 0.1 m2/g, whereas the surface area of organic polymer monolith with methacrylic acid increased significantly up to 261 m2/g. The addition of methacrylic acid in to the polymerization mixture improves also separation power of prepared monolithic columns. Figure 1 shows the separation of the mixture of small molecules on the column without (A) and with (B) methacrylic acid in the polymerization mixture.

 Usually, I leave instrument’s computer up and running for long periods of time. No surprise that after several weeks there is significant amount of Windows Updates ready for installation. This week I had to reboot my system (Dionex Ultimate 3000) and during this occasion several new updated were installed.
Usually, I leave instrument’s computer up and running for long periods of time. No surprise that after several weeks there is significant amount of Windows Updates ready for installation. This week I had to reboot my system (Dionex Ultimate 3000) and during this occasion several new updated were installed.